NOMID Safety
KINERET safety profile in NOMID patients
KINERET has a well-documented safety profile of clinical and patient experience in NOMID1,2
- No patient permanently discontinued KINERET treatment due to ISRs, the majority of which were mild (76%) or moderate (24%)1
- There were 24 serious adverse events (SAEs) reported in 14 of the 43 treated patients1,*
- The most common adverse events associated with KINERET were ISRs1
*The most common SAEs were infections (a total of 13 infections in 7 patients, with pneumonia and gastroenteritis occurring in 3 and 2 patients, respectively. Five SAEs were related to lumbar puncture, which was a study procedure).1
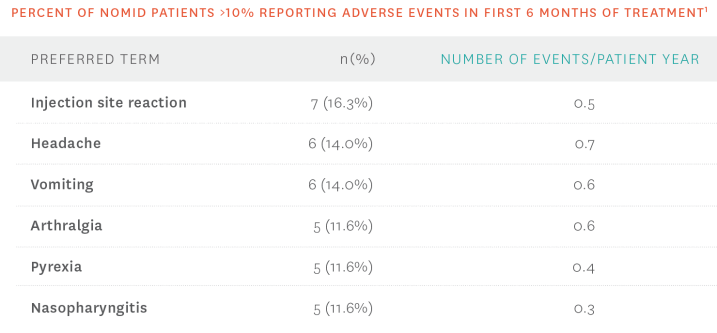
Additional safety information1
- In KINERET-treated NOMID patients, the risk of a disease flare when discontinuing KINERET treatment should be weighed against the potential risk of continued treatment. Do not initiate KINERET in patients with active infections
- Use in combination with TNF-blocking agents is not recommended
- Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported
- The impact of treatment with KINERET on active and/or chronic infections and the development of malignancies is not known
- Live vaccines should not be given concurrently with KINERET
- Neutrophil counts should be assessed prior to initiating KINERET treatment, and while receiving KINERET, monthly for 3 months, and thereafter quarterly for a period up to 1 year
