About KINERET
How KINERET works
KINERET is the recombinant form of interleukin-1 receptor antagonist (IL-1Ra), which treats autoinflammation by1:
- Supplementing naturally occurring endogenous IL-1Ra
- Competitively binding to the IL-1 type 1 receptor (IL-1R1)
- Blocking the activity of IL-1 cytokines that trigger the IL-1 inflammatory cascade
The effects of IL-1
Elevated levels of IL-1 may be responsible for symptoms such as:
- Cartilage degradation2
- Bone resorption2,3
- Other autoinflammatory symptoms4-7
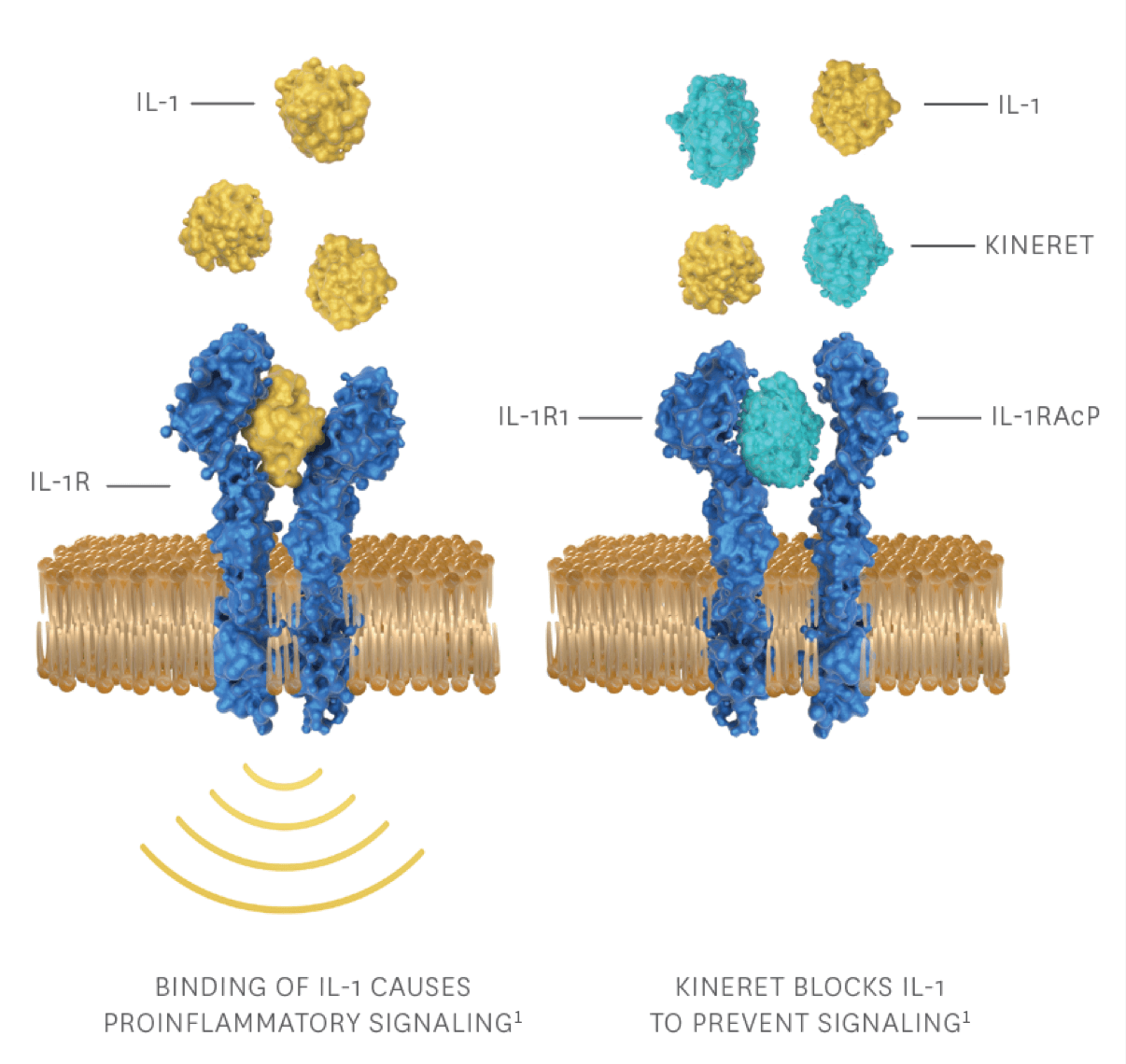
IL-1RAcP, interleukin-1 receptor accessory protein.

View the mechanism of action video to see how KINERET works
Learn about KINERET for RA patients.
For RA patientsLearn about KINERET for NOMID patients.
For NOMID patientsLearn about KINERET for DIRA patients.
For DIRA patientsReferences
- KINERET [Prescribing Information]. Stockholm, Sweden: Swedish Orphan Biovitrum AB (publ).
INDICATIONS
KINERET® (anakinra) is an interleukin-1 receptor antagonist indicated for:
Rheumatoid Arthritis (RA). Reduction in signs and symptoms and slowing the progression of structural damage in moderately to severely active rheumatoid arthritis, in patients 18 years of age or older who have failed 1 or more disease-modifying antirheumatic drugs (DMARDs). KINERET can be used alone or in combination with DMARDs other than tumor necrosis factor (TNF)-blocking agents
Cryopyrin-Associated Periodic Syndromes (CAPS). Treatment of Neonatal-Onset Multisystem Inflammatory Disease (NOMID)
Deficiency of Interleukin-1 Receptor Antagonist (DIRA). Treatment of Deficiency of Interleukin-1 Receptor Antagonist (DIRA)
CONTRAINDICATION
KINERET is contraindicated in patients with known hypersensitivity to E. coli–derived proteins, KINERET, or to any components of the product.
IMPORTANT SAFETY INFORMATION
Serious Infections. KINERET has been associated with an increased incidence of serious infections in clinical trials in RA. In RA, discontinue use if serious infection develops. In NOMID or DIRA patients, the risk of disease flare when discontinuing KINERET treatment should be weighed against the potential risk of continued treatment. Do not initiate KINERET in patients with active infections.
IL-1 blocking drugs such as KINERET may increase the risk of tuberculosis (TB) or other opportunistic infections.
Use in combination with tumor necrosis factor (TNF)-blocking agents is not recommended due to potential for increased rate of serious infections.
Hypersensitivity reactions, including anaphylactic reactions and angioedema, and serious cutaneous reactions including drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported. Patients with DIRA may have an increased risk of allergic reactions.
For severe hypersensitivity or allergic reactions, promptly discontinue KINERET and treat appropriately.
Immunosuppression. The impact of treatment with KINERET on active and/or chronic infections and the development of malignancies is not known.
Amyloidosis. There have been post-marketing reports of injection site amyloid deposits, and in some cases systemic AIL1RAP (IL-1 receptor antagonist protein) amyloidosis. Recommend patients to rotate their injection sites. Monitor proteinuria for systemic amyloidosis in patients with confirmed injection site amyloid deposits.
Immunizations. Live vaccines should not be given concurrently with KINERET.
Decreases in neutrophil counts may occur with KINERET treatment. Assess neutrophil counts prior to initiating KINERET treatment, and while receiving KINERET, monthly for 3 months, and thereafter quarterly for a period up to 1 year.
Serious Adverse Reactions
RA: The most serious adverse reactions were serious infections and neutropenia, particularly when used in combination with TNF-blocking agents.
NOMID and DIRA: The most serious adverse events were infections.
Most Common Adverse Reactions
RA: The most common adverse reactions (≥5%) are injection site reaction, worsening of rheumatoid arthritis, upper respiratory tract infection, headache, nausea, diarrhea, sinusitis, arthralgia, flu-like symptoms, and abdominal pain.
NOMID: The most common AEs during the first 6 months of treatment (>10%) are injection site reaction, headache, vomiting, arthralgia, pyrexia, and nasopharyngitis.
DIRA: The most common AEs are upper respiratory tract infections, rash, pyrexia, influenza-like illness, and gastroenteritis.
Post-marketing Experience
Hepato-biliary disorders (elevations of transaminases; non-infectious hepatitis), thrombocytopenia, including severe thrombocytopenia, and DRESS have been identified during postapproval use of KINERET. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
References: 1. KINERET (anakinra) prescribing information. Stockholm, Sweden: Sobi, Inc. 2025. 2. Arend WP, Dayer J-M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33(3):305-315. 3. van den Berg WB. Arguments for interleukin 1 as a target in chronic arthritis. Ann Rheum Dis. 2000;59(suppl 1):i81-i84. 4. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720-3732. 5. Vela P. Extra-articular manifestations of rheumatoid arthritis, now. Eur Med J Rheumatol. 2014;1:103-112. 6. Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633-652. 7. Hashkes PJ, Toker O. Autoinflammatory syndromes. Pediatr Clin North Am. 2012;59(2):447-470.
