NOMID
KINERET for NOMID
She’s one of a kind. So is her treatment
KINERET is the first and only FDA-approved treatment for neonatal-onset multisystem inflammatory disease (NOMID).1
Prescribe KINERETNOMID is a rare, predominantly pediatric autoinflammatory disease2
NOMID can be challenging to diagnose and can cause long-term disability without treatment.2
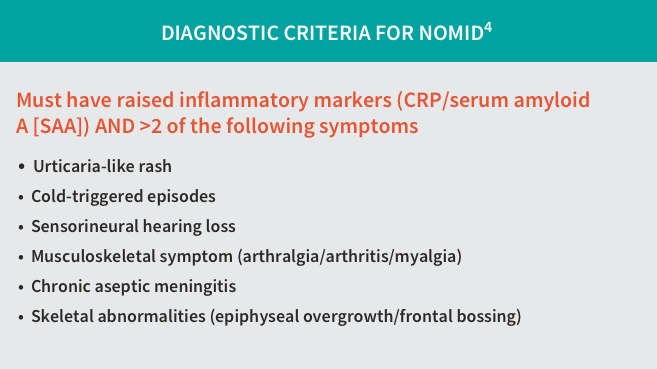
- A diagnosis is derived from inflammatory markers, clinical symptoms, severity, and age of onset3,4
- Symptoms of NOMID include chronic aseptic meningitis, urticaria-like rash, fever, vomiting, joint pain, headache, and conjunctivitis4
- Over time, chronic inflammation can lead to developmental delay, sensorineural hearing loss, physical disability, and intellectual disability5
- Although NOMID is often associated with mutations in the CIAS1/NLRP3 gene, approximately 40% of NOMID patients test negative using conventional genetic analyses6
- 2016 clinical diagnostic criteria for CAPS do not “mandate evidence of a disease-causing NLRP3 mutation”4


The degenerative, autoinflammatory nature of NOMID can cause irreversible damage, so early identification is vital.3
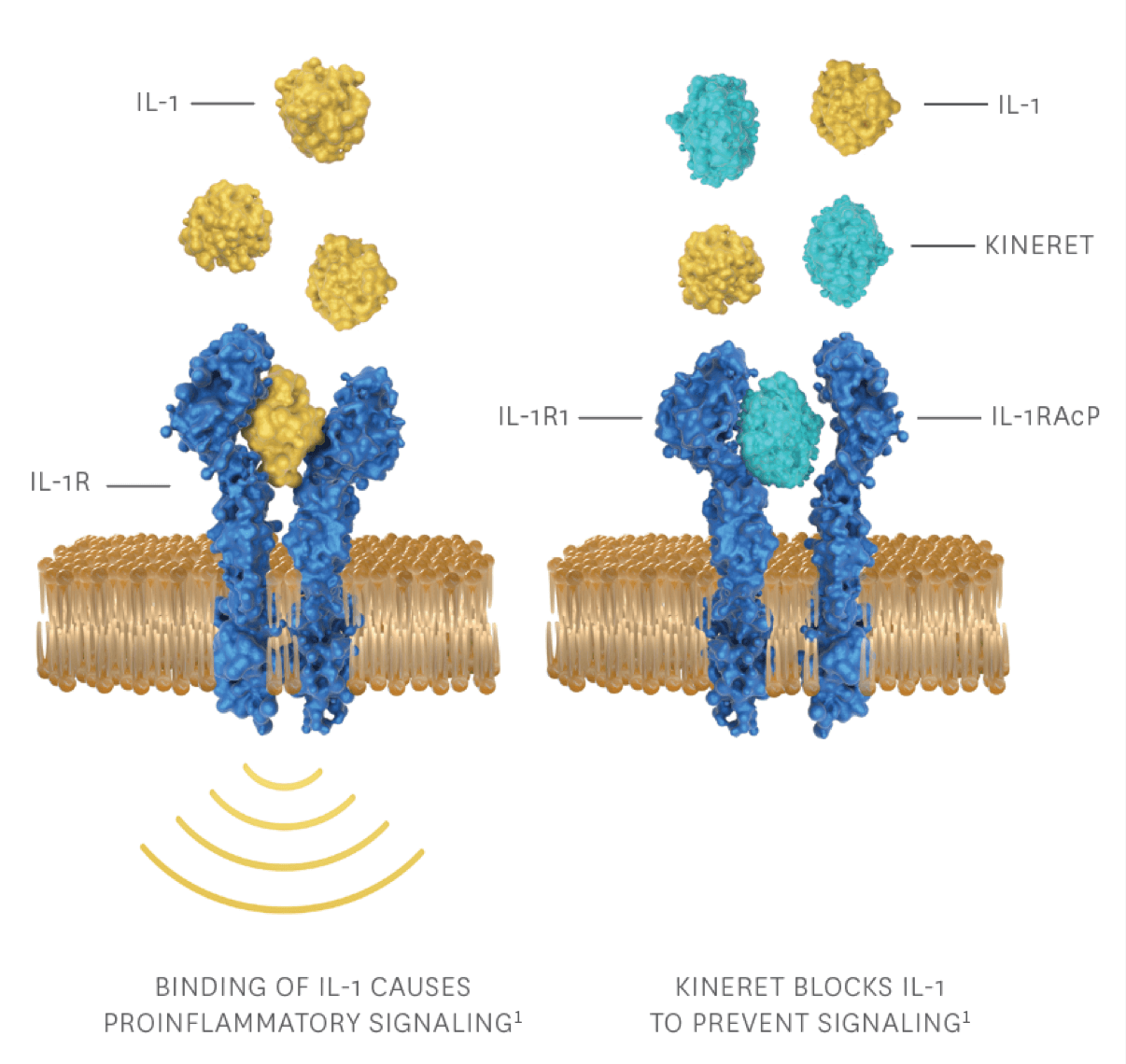
Autoinflammation in NOMID is driven by IL-12
- IL-1 (IL-1α and IL-1β) is a prototypic proinflammatory cytokine7
- Secretion of IL-1β has an important role in systemic inflammation and in the signs and symptoms of NOMID1
- NOMID is often associated with mutations in the CIAS1/NLRP3 gene, which encodes the protein cryopyrin (NLRP3), an important component of the NLRP3 inflammasome8,9,*
- Although approximately 40% of patients test negative for CIAS1/NLRP3 using conventional genetic analyses, advanced testing techniques have identified somatic NLRP3 mosaicism in ~70% of these patients6
- A mutation in cryopyrin leads to an increased rate of inflammasome assembly without the necessary inflammatory stimuli3
- Increased inflammasome activity results in excessive production of the proinflammatory cytokine IL-1β3,8
*A multiprotein complex that activates inflammatory responses.