RA Efficacy
An in-depth look at the data in RA
KINERET was studied in 899 patients with active RA1
This randomized, double-blind, placebo-controlled trial was conducted on 899 patients with active RA who had been on a stable dose of methotrexate (10-25 mg/week) for at least 8 weeks. Patients were randomized to KINERET (n=250) or placebo (n=251) in addition to their stable doses of methotrexate. The first 501 patients were evaluated for signs and symptoms of active RA.
*17% + 6% = 23%.
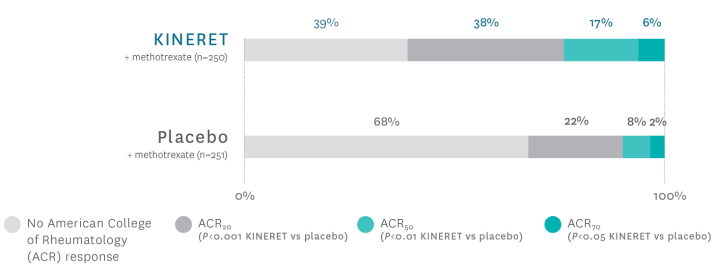
Patients using KINERET saw improvements in ACR component scores1
In addition to swollen, tender, or painful joints, elevated ESR and CRP levels can indicate systemic autoinflammation, which KINERET has been shown to control in refractory RA patients.
A 24-week study was conducted in 242 patients with active RA on background methotrexate who were randomized to receive either etanercept alone or the combination of KINERET and etanercept. The ACR50 response rate was 31% for patients treated with the combination of KINERET and etanercept and 41% for patients treated with etanercept alone, indicating no added clinical benefit of the combination over etanercept alone. Serious infections were increased with the combination compared to etanercept alone.

At 6 months, KINERET DEMONSTRATED REDUCTION IN OBJECTIVE MEASURES like acute-phase reactant levels1:
- ESR decreased by nearly half (47.2%) from baseline
- CRP levels decreased by 77% from baseline
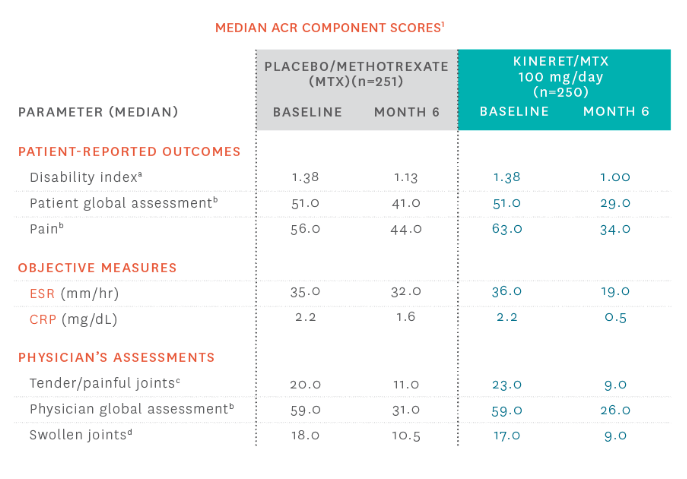
aHealth Assessment Questionnaire (HAQ); 0 = best, 3 = worst; includes 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities. bVisual analog scale; 0 = best, 100 = worst. cScale 0 to 68. dScale 0 to 66.

At 6 months, KINERET DEMONSTRATED REDUCTION IN OBJECTIVE MEASURES like acute-phase reactant levels1:
- ESR decreased by nearly half (47.2%) from baseline
- CRP levels decreased by 77% from baseline
Radiographic changes improved over the course of 1 year with KINERET1
The effect of KINERET on the progression of structural damage was assessed by measuring the change from baseline at month 12 in the TMSS and its subcomponents, erosion score, and JSN score. Radiographs of hands/wrists and forefeet were obtained at baseline, 6 months, and 12 months and scored by readers who were unaware of treatment group. Differences between placebo and KINERET for change in TMSS, ES, and JSN score were observed at 12 months.
The disability index of the HAQ was administered monthly for the first 6 months and quarterly thereafter during the study.
Health outcomes were assessed by the Short Form-36 (SF-36) questionnaire. The 1-year data on HAQ in Study 1 showed more improvement with KINERET than placebo. The physical component summary score of the SF-36 also showed more improvement with KINERET than placebo but not the mental component summary.
Prescribe KINERET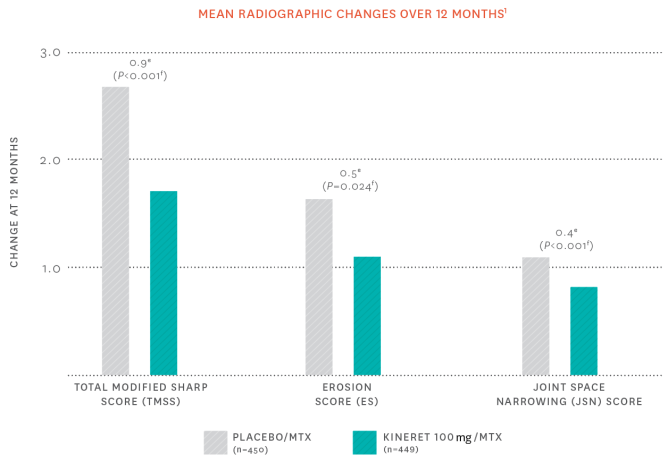
eDifferences and 95% confidence intervals for the differences in change scores between placebo/MTX and KINERET/MTX. fBased on Wilcoxon rank sum test. MTX, methotrexate.

Following 1 year of treatment, TMSS CHANGES WERE 42% LESS IN KINERET PATIENTS compared with placebo.1
